Four Months of Momentum: Adhera Health’s Breakthroughs in Empathic AI, Clinical Evidence, and Industry Recognition
From ENDO 2025 to DTM to AIMed25, Adhera Health has been on a remarkable trajectory. Our research was featured by the Endocrine Society, our AI Health Agentic Platform drew strong interest from diabetes innovators, and our empathic AI approach won First Place at AIMed25’s Shark Tank competition. This momentum signals a shift: family-centered, emotionally intelligent AI is emerging as the future of pediatric chronic care.
The past several months has been a remarkable period of growth, validation, and recognition for Adhera Health. From publishing three peer-reviewed studies to presenting at ENDO 2025, participating in the Diabetes Technology Meeting’s Startup Showcase, and winning the AIMed25 Shark Tank competition - one theme has been consistent: Empathic AI is resonating across the entire healthcare ecosystem.
This momentum represents years of work building emotionally intelligent, family-centered technologies that support families, clinicians, and care teams in pediatric chronic care.
Three New Peer-Reviewed Studies in JMIR Pediatrics & Parenting
This year, we published three studies in JMIR Pediatrics & Parenting validating the impact of our digital programs across pediatric chronic conditions.
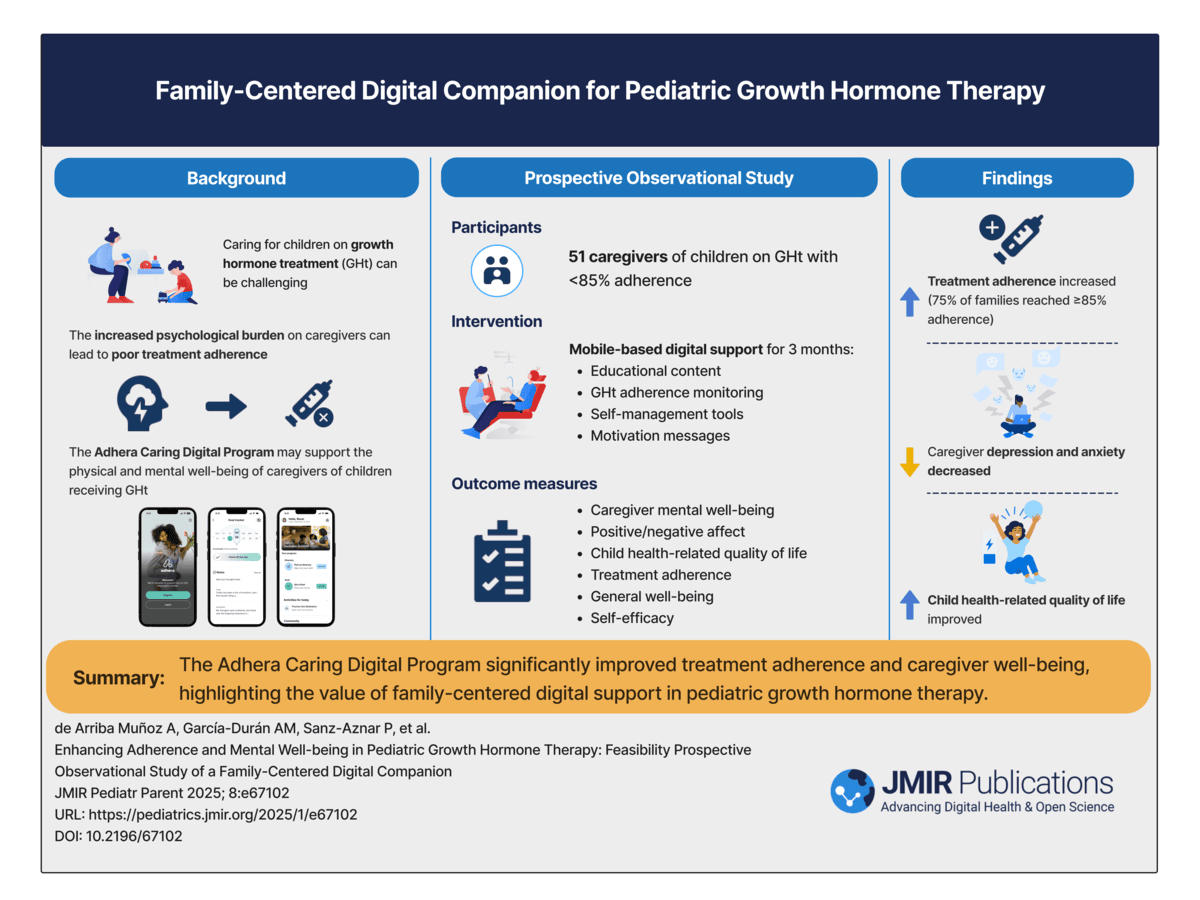
1. Enhancing Adherence and Mental Well-being in Pediatric Growth Hormone Therapy
This study demonstrated improvements in adherence, emotional well-being, and confidence among families using a family-centered digital companion during pediatric GHD therapy.
JMIR Pediatrics & Parenting Published on 27.Oct.2025
This is a visual abstract summarizing the key findings of the research titled "Enhancing Adherence and Mental Well-Being in Pediatric Growth Hormone Therapy: Feasibility Prospective Observational Study of a Family-Centered Digital Companion," published in JMIR Pediatrics and Parenting in 2025. The study found that the Adhera Caring Digital Program significantly improves treatment adherence and enhances caregivers’ quality of life.
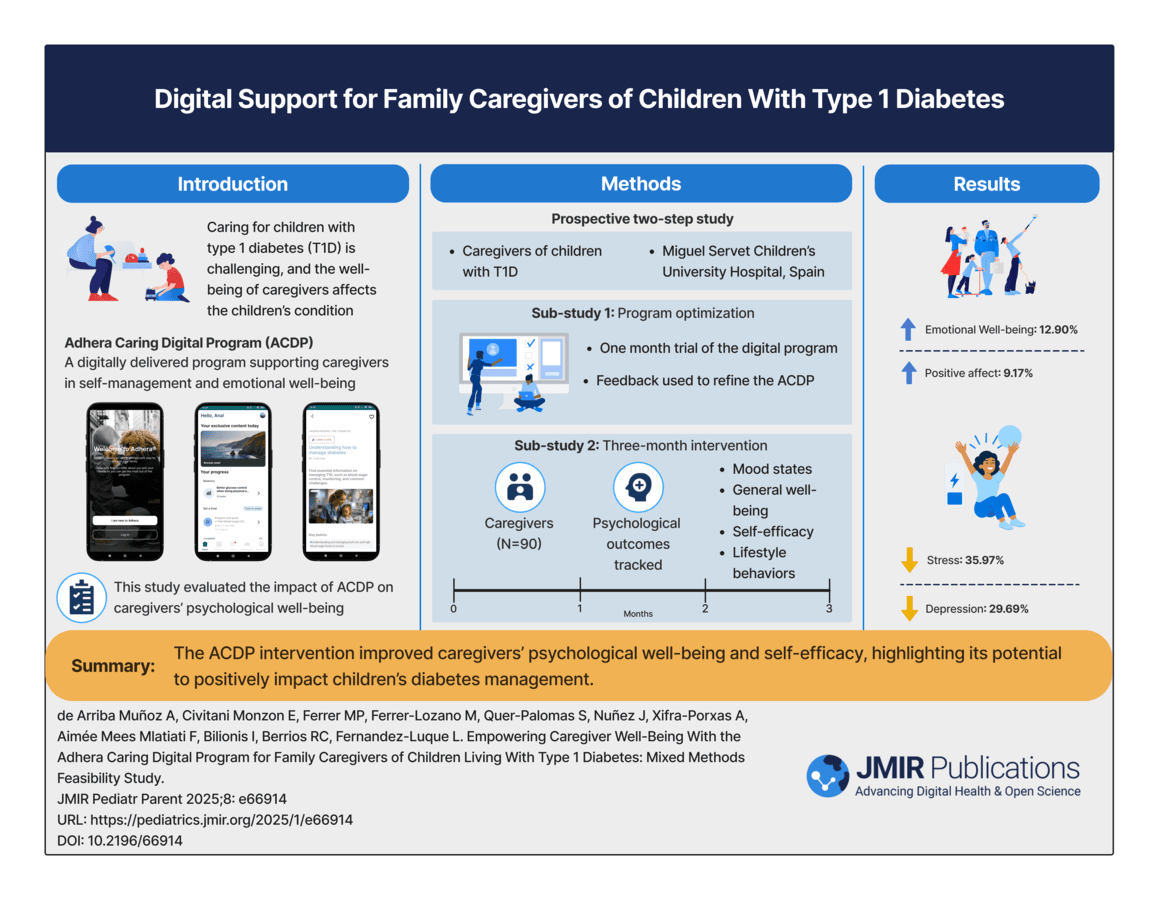
2. Empowering Caregiver Well-Being for Families Managing Type 1 Diabetes
This publication showed meaningful reductions in caregiver depression and anxiety, along with improved confidence and treatment adherence.
JMIR Pediatrics & Parenting Published on 05.Feb.2025
This is a visual abstract summarizing the key findings of the research titled "Empowering Caregiver Well-Being With the Adhera Caring Digital Program for Family Caregivers of Children Living With Type 1 Diabetes: Mixed Methods Feasibility Study," published in JMIR Pediatrics and Parenting in 2025. The study found that the Adhera Caring Digital Program improved caregivers’ psychological well-being and self-efficacy, suggesting that digital solutions can support caregiver mental health and enhance diabetes management.
3. Digital Health Support for Caregivers of Children Undergoing Growth Hormone Therapy
This qualitative analysis revealed emotional and behavioral benefits for caregivers supporting children through growth hormone therapy.
JMIR Pediatrics & Parenting Published on 05.Feb.2025
This is a visual Abstract of the paper titled: Digital Health Program to Support Family Caregivers of Children Undergoing Growth Hormone Therapy: a Qualitative Feasibility Study.
Scientific Leadership at ENDO 2025
Our Growth Hormone Disorder study, conducted with the Pediatric Endocrinology Department at Hospital Miguel Servet and supported by Merck, was selected both as a poster and for the highly competitive Rapid Fire format. Presented by Luis Fernández Luque, PhD, Adhera Health’s Chief Scientific Officer, the research was also chosen as one of just 24 abstracts (out of more than 2,700) featured in the Endocrine Society’s official press releases. The study emphasized the strong relationship between caregiver mental health and a child’s coping, adherence, and clinical trajectory - reinforcing the need for holistic, family-centered interventions that account for the shared emotional experience of GHD.
Our T1D presentation, supported by Novo Nordisk, explored how severe hypo- and hyperglycemic events - especially at night - impact not only children’s well-being but also caregiver stress and children’s academic performance.
And through our NIH-funded partnership with Children’s Hospital Los Angeles, we brought forward the lived experiences of Latino families managing childhood obesity, revealing cultural, emotional, and structural challenges that point to an urgent need for more culturally competent, emotionally supportive tools.
Together, these studies reflect Adhera Health’s growing scientific momentum and reinforce our commitment to advancing emotionally intelligent, family-centered digital support across pediatric chronic conditions.
Startup Showcase at the 2025 Diabetes Technology Meeting
On October 28, Adhera Health was featured in the Startup Showcase at the Diabetes Technology Meeting (DTM), hosted by the Diabetes Technology Society and co-sponsored by the American Diabetes Association. Being selected for this stage allowed us to share our growing body of scientific evidence and highlight the real-world needs of families managing complex pediatric chronic conditions.
At DTM, we presented our evolution from the Adhera Caring Digital Program (ACDP) to our AI Health Agentic Platform, illustrating how emotionally intelligent, family-centered AI can extend clinical care into the home. We demonstrated how our agents support families navigating Type 1 Diabetes and obesity/pre-diabetes, addressing not only treatment adherence but also the behavioral, emotional, and cultural factors that shape a child's health journey. This milestone further reinforced our momentum as we continue to advance next-generation digital tools that meet families where they are.
AIMed25 Shark Tank Winner
And just last week, Adhera Health achieved one of our most exciting milestones yet - winning First Place at the AIMed25 Shark Tank competition in San Diego, California. Competing among some of the most innovative AI-driven healthcare companies, our vision for family-centered pediatric chronic care stood out clearly to the judges.
They highlighted the strength of our approach, including:
Emotionally intelligent AI Health Agents that extend compassionate support into the home
Small Language Models (SLMs) trained on real whole-family data
Clinically validated impact, backed by peer-reviewed studies and real-world evidence
A family-centered design philosophy that supports children, caregivers, clinicians, and care teams
This recognition underscores what we believe deeply: Empathic AI isn’t just a technological advance - it’s the foundation of modern pediatric chronic care.
A Unified Digital Ecosystem
Adhera Health connects families, clinicians, care teams, payors, and employers in a unified digital ecosystem that bridges clinical care and real-world family life.
Our AI Health Agents enable:
Family-centered pediatric chronic care management
Emotionally intelligent support in the home
Improved adherence, engagement, and caregiver well-being
Actionable insights for health systems and employers
Better coordination across the care continuum
By linking clinical outcomes to the day-to-day lives of families, our platform reduces both the human and financial burden of pediatric chronic conditions.
Conclusion
This season of recognition - three JMIR studies, ENDO presentations, DTM Showcase participation, and the AIMed25 win - marks a defining moment for Adhera Health.
We set out to build technology rooted in empathy and science. Today, with growing validation across research, clinical communities, and innovation platforms, that vision is taking shape in powerful ways.
Empathic AI isn’t the future - it’s what families need right now.
Standing With California Families: What I’ve Learned From Parents Navigating Type 1 Diabetes
Managing a child’s type 1 diabetes brings more than medical challenges — it brings emotional, social, and financial strain for families across California. Through Adhera Health’s T1D beta program, I’ve heard directly from parents about their struggles and their hopes. One mother told me, “Letting out my voice gives me relief.” Another shared, “I felt love from people I didn’t even know.” These voices remind us why our AI + human digital companion must combine empathy, education, and community to truly support families.
In California, thousands of families live with the daily realities of pediatric type 1 diabetes (T1D). Nationally, more than 300,000 children and adolescents under 20 have T1D, with nearly 20,000 new cases diagnosed each year (source). For parents, the diagnosis brings not just medical complexity but also emotional and social challenges - from sleepless nights to stigma at school.
At Adhera Health, we are AI-native, building companions that combine intelligent digital agents with human coaching and community support. Our flagship, the Adhera Caring Digital Program (ACDP), delivers evidence-based, personalized support for families managing pediatric chronic conditions.
Recently, we launched our T1D beta program, and I had the privilege of interviewing parents participating in it. Their voices continue to guide how we design meaningful support.
What I’ve learned from these conversations:
Emotional support matters deeply. Parents often described overwhelm, grief, and fear after diagnosis. One mother told me, “Letting out my voice gives me relief.” Another said, “This came at the right time after a very dark moment.”
Education is power. Families praised step-by-step guidance on CGM use, carb counting, and school communication. Bite-sized lessons were described as “clear and easy” and “more than I expected.”
Community brings comfort. When caregivers connected with peers, the impact was profound. “I felt love from people I didn’t even know,” one parent shared. That sense of belonging is something no algorithm alone can provide - it’s the human layer that makes AI support real.
Families want child-inclusive tools. Parents of older children voiced a desire for features that help kids take more responsibility, building both confidence and independence.
These reflections align with what our peer-reviewed research in JMIR Pediatrics & Parenting has shown: family-centered digital interventions can reduce caregiver stress and improve adherence in pediatric chronic care:
Our beta shows that impact is not just theoretical - it’s happening in real homes, with real families.
As we deepen partnerships with California payors, children’s hospitals, and advocacy groups, our goal remains clear: support families with tools that combine the best of AI and human care, improve outcomes for children, and lower long-term healthcare costs.
I’m also excited to share that we are preparing to launch our beta program for families managing pediatric obesity, further expanding how we support families across conditions. And later this month, I’ll be attending the California Association of Health Plans Annual Conference in Desert Springs, CA, meeting with executives from California health plans, Medi-Cal, and others to discuss the results we’ve been seeing with families - and what’s next.
Adhera Health at ENDO 2025: Championing Family-Centered Innovation in Pediatric Chronic Care
At ENDO 2025, Adhera Health presented three groundbreaking studies on pediatric chronic conditions—highlighting how AI-powered digital companions can improve both child and caregiver outcomes in families managing growth hormone disorders, type 1 diabetes, and obesity. Here’s what we shared, who we partnered with, and why it matters.
Last week at ENDO 2025 in San Francisco, Adhera Health took the stage with three research presentations focused on family-centered approaches to pediatric chronic condition care. The team presented posters on Growth Hormone Deficiency (GHD), Type 1 Diabetes (T1D), and Pediatric Obesity - and delivered a Rapid Fire presentation during the Pediatric Growth Hormone session on Day 1.
Mental & Emotional Health in Growth Hormone Disorders
Ricardo C. Berrios, Adhera Health’s CEO and Luis Fernandez Luque, PhD, Adhera Health’s Chief Scientific Officer, presenting at ENDO 2025
Our study on GHD, presented in both poster and rapid fire formats, emphasized the interconnected emotional and physical challenges experienced by children and their caregivers. It highlighted how caregiver mental health is strongly associated with a child’s coping and treatment outcomes. These findings reinforce the need for holistic, family-centered interventions that address the full lived experience of GHD - not just the medical aspects. This study was conducted in partnership with the University of Miguel Servet’s Pediatric Endocrinology Department (led by Dr. Antonio de Arriba) and supported by Merck GmbH.
From over 2,700 abstracts presented at ENDO 2025, only 24 were selected for official Endocrine Society press releases - and our study was one of them. You can read the release here.
Our findings didn’t just make waves at ENDO — they also sparked coverage in leading science and health media outlets like News-Medical and Medical Xpress, helping to bring the conversation around family-centered pediatric care to a broader global audience.
Luis Fernandez Luque, PhD, Adhera Health’s Chief Scientific Officer, presenting at ENDO 2025
Type 1 Diabetes and Severe Glycemic Events
Our second presentation, titled "Associations Between Severe Events of Hypo- and Hyperglycemia and Family Well-being", explored how fluctuations in blood sugar levels - particularly at night - affect not just children with T1D, but also their caregivers’ emotional state and children’s academic performance. Caregiver education emerged as a key protective factor, reinforcing the value of empowering families with knowledge and emotional guidance. This study was conducted in partnership with the University of Miguel Servet’s Pediatric Endocrinology Department (led by Dr. Antonio de Arriba) and supported by Novo Nordisk.
Obesity in Underserved Families
Our third poster focused on the lived experiences of Latino families managing childhood obesity. Through 11 participatory workshops involving over 50 families, the study uncovered key challenges: stigma, cultural food practices, limited access to resources, and mental health gaps. Caregivers stressed the need for culturally competent, emotionally supportive tools - pointing to digital companions as a promising solution to close the care gap. This work was supported by an SBIR grant from the NIH in partnership with Jennifer Raymond, MD, MCR an Associate Professor in Pediatric Endocrinology at Children's Hospital Los Angeles and her research team.
Shannon McGinley, Adhera Health’s Director of Marketing and one of the researchers on the NIH-funded study
Collaborator Connections at ENDO
Beyond the research presentations, ENDO 2025 was an opportunity to deepen our partnerships. We met with critical Key Opinion Leaders including Dr. Brad Miller (Professor of Pediatrics, University of Minnesota) and Dr. William Martinez ((Associate Professor of Clinical Psychiatry and Behavioral Sciences, University of California, San Francisco) to discuss future directions for research in growth disorders and mental health in pediatric obesity. We also connected with Dr. Tina Hu, Clinical Director of Diabetes Transition of Care at UCSF, to explore the evolving needs of adolescents with diabetes as they age into adult care.
Ricardo C. Berrios, Luis Fernandez Luque and Dr. William Martinez, Associate Professor of Clinical Psychiatry, UCSF, discussing future partnerships
Looking Ahead
We’re proud to be advancing the science behind family-centered care in pediatrics. Whether it’s Growth Hormone Deficiency, Type 1 Diabetes, or Obesity, our work highlights the power of pairing digital innovation with human understanding - and bringing the whole family into the center of chronic care management.
View the Posters:
Adhera Health Research Spotlighted at ENDO 2025: Advancing AI-Driven Family-Centered Pediatric Care
Adhera Health’s latest research, featured at ENDO 2025, explores how AI-driven digital companions can support families managing pediatric chronic conditions like T1D, GHD, and obesity - putting science and empathy at the heart of care.
We’re thrilled to share that three of Adhera Health’s research studies have been accepted for presentation at ENDO 2025, the annual meeting of the Endocrine Society and one of the world’s leading forums for endocrine innovation. Taking place this July in San Francisco, the event will spotlight how our work is reshaping pediatric chronic care through clinically grounded, AI-powered solutions that support not just the child - but the entire family.
Groundbreaking Research, Family-First Impact
At Adhera Health, our mission is rooted in one fundamental belief: pediatric care must be family-centered to be truly effective. The studies selected for ENDO 2025 dive into the emotional, behavioral, and systemic realities of families navigating three complex conditions:
Growth Hormone Deficiency: A study revealing the deep interdependence between child and caregiver well-being.
Type 1 Diabetes (T1D): Research examining how AI can help identify patterns in hypo- and hyperglycemic events to better support families.
Childhood Obesity in Underserved Populations: A participatory initiative engaging Latino families to uncover cultural and systemic barriers, shaping more inclusive digital support tools.
“These insights are more than scientific findings - they’re the foundation of how we build technology that actually works for families,” said Ricardo C. Berrios, Co-Founder & CEO of Adhera Health. “We’re honored to share this work with the broader endocrine community at ENDO 2025.”
Powering the Future with Adhera AI Health Agents
These studies directly inform the development of the Adhera® AI Health Platform and our proprietary Adhera Health Agents - goal-oriented, autonomous AI companions built on Small Language Models (SLMs) and fueled by biopsychosocial family data. These agents are designed to support the entire care journey, offering timely, empathetic, and personalized guidance to families navigating chronic conditions.
“Our collaboration with Adhera Health shows how digital innovation must be both scientifically sound and human-centered,” said Dr. Antonio de Arriba Muñoz, Pediatric Endocrinologist at Hospital Universitario Miguel Servet.
Scaling Empathy and Science
As we scale nationally, these findings reinforce our belief that the next generation of healthcare tools must go beyond automation. They must understand, support, and connect—in ways that are equitable, emotionally intelligent, and clinically aligned.
“This is AI that doesn’t just think—it understands,” Berrios added. “It’s not just advanced, it’s deeply empathetic.”
To learn more about our work at Adhera Health and how we’re transforming pediatric chronic care, get in touch with us.
This blog article is based on the press release - https://www.einpresswire.com/article/819470694/adhera-health-strengthens-ai-driven-pediatric-platform-with-research-featured-at-endo-2025
Meet Connie Dovel: A Health & Wellness Coach Who Gets It
“It’s not just about what to eat or how to dose. It’s about listening - really listening - and working together to find what fits.”
Connie Dovel, Health and Wellness Coach for the Adhera Caring Digital Program, knows firsthand what families go through when a child is diagnosed with Type 1 diabetes. As a parent of a T1D child herself, she brings deep empathy, real-life experience, and evidence-based support to every coaching interaction. In this blog, Connie shares how she helps families feel more confident, supported, and seen - one day at a time.
At Adhera Health, we believe that caring for a child with Type 1 diabetes takes more than clinical appointments - it takes everyday courage, emotional support, and a sense of being truly understood. That’s exactly what families find in Connie Dovel, one of the compassionate Health and Wellness Coaches behind the Adhera Caring Digital Program.
Connie’s role goes far beyond advice - it’s about connection, empathy, and empowerment. Here’s a closer look at what fuels her work and how she supports families navigating pediatric Type 1 diabetes.
A Lifelong Passion for Health - and a Personal Connection to T1D
Connie’s career in health and wellness spans personal training, nutrition counseling, and health promotion. With a Bachelor’s in Exercise Science, a Master’s in Health Promotion Management, and certifications in both personal training and health coaching, she brings deep knowledge to every coaching session.
But what makes her truly unique is that she’s walked this road herself:
“My oldest child was diagnosed with Type 1 diabetes at 11. I can empathize with and guide families because I’ve faced the same challenges they’re facing every single day.”
Coaching That’s Personal, Not One-Size-Fits-All
Through the Adhera app, Connie supports families with:
One-on-one chat support
Scheduled video sessions
Each conversation is tailored to a family’s unique concerns. Whether it’s navigating sports safely, managing picky eating, or adjusting insulin around busy schedules, Connie adapts to meet them where they are.
“Every interaction is different. There’s no script. Just a real conversation based on what that family needs right now.”
A Moment That Meant Everything
Connie recalls supporting a teen girl with T1D who was completely burned out and disengaged from her care.
“She had given up. Her parents were overwhelmed. We took small steps, little by little. Months later, she messaged me:
‘Thank you for believing in me when I didn’t believe in myself.’
That’s why I do this.”
Tools That Help Families Feel in Control
Connie often begins with goal setting - short-term, long-term, and everything in between. From mindfulness exercises to daily check-ins, each strategy is customized. And thanks to the app’s chat function, support fits into real life, not just a calendar.
“Families are busy. The digital connection means we don’t need to wait for a scheduled session. We can talk when it matters most.”
Inclusive, Accessible Support for Every Family
Connie works with families from all backgrounds. Her approach is grounded in respect, curiosity, and flexibility, with sensitivity to cultural, social, and emotional differences.
“It’s not just about what to eat or how to dose. It’s about listening - really listening - and working together to find what fits.”
Looking Ahead with Adhera Health
As someone who once wished for this kind of support herself, Connie is energized by where Adhera is headed:
“This concept is revolutionary. I wish something like this existed when my child was diagnosed. I’m honored to be part of it.”
Her advice to every caregiver?
“Take it one day at a time. Don’t be afraid to accept help. And remember—every small win matters.”
Learn More About the Adhera Caring Digital Program
Discover how Adhera Health supports families managing pediatric chronic conditions with empathy, evidence, and everyday tools that work.
What AMIA 2025 Taught Adhera Health About Fair, Responsible AI in Healthcare
What AMIA 2025 Taught Us About Fair, Responsible AI in Healthcare
At the AMIA Informatics Summit 2025, Adhera Health shares our vision for Precision Digital Companions that prioritize fairness, equity, and real-world family needs. From tackling bias in AI models to integrating Social Drivers of Health (SDoH), we walked away with powerful insights that are shaping how we design digital health solutions for children with chronic conditions - and their caregivers.
Read the full reflection from our Lead Data Scientist, Ioannis Billions, and learn how these lessons are fueling our next phase of innovation.
Insights from the AMIA Informatics Summit that are helping guide our approach to using AI and digital tools to better support families managing chronic health conditions.
Earlier this month, I had the opportunity to attend and present at the AMIA Informatics Summit 2025, one of the most influential conferences in health informatics. The event brought together leading researchers, clinicians, and industry innovators to explore the latest advancements in AI, machine learning, and digital health solutions.
Attending this summit was essential for Adhera Health as we continue advancing our mission in digital health innovation. Our goal is to develop Precision Digital Companions that leverage AI, machine learning, and Social Determinants of Health (SDoH) to improve health outcomes—especially for families managing pediatric chronic conditions, a group often overlooked in digital health innovation. The discussions at AMIA reinforced the importance of fairness in AI, ethical deployment of machine learning models, and the critical role of SDoH in shaping equitable healthcare solutions.
Key Takeaways from the Summit
1. Fairness in AI: Addressing Bias in Machine Learning for Chronic Disease Care
One of the most pressing challenges in AI-driven healthcare is the issue of bias in machine learning models. During my presentation, I highlighted how current AI models often favor younger, male patients, leading to disparities in chronic disease care. These biases, embedded in training data, can result in less accurate predictions for older adults, women, and underrepresented populations—widening healthcare inequalities.
The session sparked thought-provoking discussions with attendees on ways to mitigate these biases, including:
1. Rebalancing training datasets to ensure diverse representation.
2. Developing fairness-aware algorithms that actively correct disparities.
3. Integrating SDoH data to capture a more holistic view of patient health.
We also discussed how fairness in AI extends beyond individual patients to their families—particularly in pediatrics, where caregivers play a central role in daily disease management. Supporting equitable outcomes means considering not just clinical risk factors, but also the emotional, logistical, and behavioral burden placed on families.
2. Engaging with Leading Experts in Biomedical Informatics
One of the most valuable aspects of AMIA is the opportunity to connect with top researchers and thought leaders. I had insightful conversations with experts from UCSF, Stanford, NYU Langone, and Mayo Clinic on cutting-edge developments in AI and health informatics, particularly in:
1. The potential and risks of Large Language Models (LLMs) in healthcare. While LLMs hold promise for patient communication and clinical decision support, concerns around hallucinations and bias remain critical.
2. Ethical considerations in AI deployment. How can hospitals ensure that AI-driven decisions align with patient-centric care?
3. Advancing NLP and machine learning in EHRs. How can we leverage AI to extract meaningful insights from electronic health records while maintaining privacy and fairness?
These discussions reinforced the importance of responsible AI development and the need for cross-sector collaboration to ensure these technologies truly benefit patients.
3. Spotlight on SDoH and AI Integration
A key theme throughout the summit was the integration of SDoH in AI models. In a discussion with a researcher from a leading children's hospital, we explored the role of SDoH data in family-centered precision digital health solutions for pediatric chronic conditions—a critical yet often overlooked aspect of AI in healthcare. These conversations reinforced the importance of designing tools that recognize caregivers as active participants in care and address the real-world challenges families face, such as housing instability, food insecurity, and access to transportation.
By incorporating factors like socioeconomic status, access to care, and environmental conditions, AI-driven tools can offer more personalized and equitable healthcare recommendations. This aligns directly with Adhera Health’s mission to create digital companion solutions that account for real-world patient contexts beyond clinical data alone.
Looking Ahead: Next Steps for Adhera Health
Insights from AMIA will directly inform the evolution of Adhera Health’s Precision Digital Companion programs. Specifically, we are focused on:
1. Enhancing AI fairness in our predictive models to reduce bias in chronic disease care.
2. Expanding partnerships with healthcare institutions and researchers to drive responsible AI innovation.
3. Continuing to refine our support for family dyads—both the child and caregiver—to ensure our digital companions foster confidence, resilience, and health literacy at home.
4. Further integrating SDoH data to improve personalization and patient engagement in digital health solutions.
We are excited about upcoming opportunities to collaborate with leaders in AI fairness, biomedical informatics, and digital health to ensure these innovations lead to more just and effective healthcare solutions.
Conclusion
Attending AMIA Informatics Summit 2025 was a powerful reminder of the potential—and responsibility—of AI in healthcare. From addressing bias in machine learning to leveraging SDoH for more equitable family-centered care, the discussions and collaborations at this event will shape the next phase of digital health innovation.
At Adhera Health, we are committed to leading this change. If you're interested in collaborating on AI fairness, SDoH integration, or digital health solutions, let’s connect!
Stay tuned for an upcoming research publication where we dive deeper into these topics. In the meantime, follow Adhera Health for more updates on our work in responsible AI and precision digital health.
Addressing Bias in AI Models for Chronic Disease Care: Why Equitable Digital Health Matters
AI-powered healthcare should work for everyone—but new research from Adhera Health reveals significant biases in machine learning models for chronic disease care. Our latest study, set to be presented at the AMIA Informatics Summit, highlights how predictive tools often favor younger, male patients, leaving older individuals at risk of inconsistent care. Learn how we’re tackling this challenge and pioneering a more equitable future for digital health.
At Adhera Health, we are committed to advancing digital health solutions that serve all families and patients equitably. A new study from our research team, set to be presented this week at the American Medical Informatics Association (AMIA) Informatics Summit in Pittsburgh, PA, reveals significant disparities in machine learning (ML) models used for clinical decision support in diabetes and heart disease management.
AI Bias in Healthcare: The Findings
Our research, Disparate Model Performance and Stability in Machine Learning Clinical Support for Diabetes and Heart Diseases, highlights how AI-driven predictive tools often favor younger and male patients while demonstrating inconsistent performance for older individuals. The study analyzed data from over 25,000 individuals with chronic diseases and found that age- and sex-related biases persist, raising concerns about fairness in AI-powered healthcare.
This finding underscores a crucial issue in healthcare AI: merely training models on diverse data does not automatically result in equitable outcomes. Many existing ML models struggle to accommodate the complexities presented by older patients, potentially leading to misdiagnoses, inappropriate treatments, or gaps in care.
The Need for More Inclusive AI
At Adhera Health, we believe that digital health solutions should work for everyone, not just the groups most commonly represented in training data. That’s why our research introduces a novel analytical framework that assesses model fairness beyond traditional performance metrics. This approach provides a blueprint for developing more reliable and unbiased AI-driven clinical tools.
Adhera Health’s Commitment to Ethical AI
Our family-centric digital companion for pediatric chronic conditions—including Type 1 diabetes, Type 2 diabetes, and childhood obesity—leverages AI-driven personalization while addressing the social, behavioral, and systemic factors that impact health outcomes. We prioritize fairness, inclusivity, and transparency in our AI models to ensure our solutions meet the needs of diverse patient populations.
“We are excited to see these important discussions take center stage at AMIA,” said Ricardo C. Berrios, CEO and Co-Founder of Adhera Health and contributor to the published research. “This study underscores the need for digital health companies to prioritize fairness and inclusivity in AI-driven tools. By leveraging new research and ethical AI principles, we can develop more effective digital health solutions that truly improve outcomes for all family members.”
Join the Conversation at AMIA 2025
If you're attending the AMIA Informatics Summit 2025, join us for a discussion on how to create more equitable AI-driven healthcare solutions. Our findings will be presented during Session S25 | Toward Implementation: Addressing Real-World Deployments on March 12 from 1:30 PM – 3:00 PM at the Omni William Penn Hotel in Pittsburgh, PA. Ioannis Bilionis, Data Scientist at Adhera Health, will be leading the presentation.
We look forward to engaging with industry leaders, clinicians, and researchers to drive meaningful advancements in ethical AI for healthcare. Stay connected with us for updates on our research and initiatives to promote equitable digital health for all.
This blog article is based on the press release - https://www.einpresswire.com/article/792388479/adhera-health-research-reveals-bias-in-ai-models-for-chronic-disease-care
Adhera Health Pioneers Family-Focused Digital Health Solutions for Children with Chronic Conditions
Adhera Health Pioneers Family-Focused Digital Health Solutions for Children with Chronic Conditions
Reveals Transformative Research Results at ISPAD 2024
Adhera Health unveiled groundbreaking research at the International Society for Pediatric and Adolescent Diabetes (ISPAD) conference showcasing how innovative digital interventions can enhance wellbeing for families affected by Type 1 Diabetes (T1D) and childhood obesity.
The first study (NCT05483803), "Impact of a Personalized Digital Intervention on the Wellbeing of Caregivers of Children with Type 1 Diabetes and Diabetes Management," revealed a significant link between caregiver stress and the child's overall wellbeing. The findings underscore the critical importance of providing comprehensive support to the entire family in managing chronic conditions. The study enrolled 90 families of children with type 1 diabetes aged 8-15 years. Caregivers interacted with the Adhera® Caring Digital Program which emphasized education, provided motivational, gradual, and substantiable change recommendations along with a dedicated coach. Caregivers completed surveys on their own wellbeing and their child's quality of life at the start and end of the 3-month program.
Results showed a significant correlation between caregiver stress and poorer child wellbeing (r=-0.37, p<0.001), emphasizing the importance of addressing caregiver wellbeing. Post-intervention, the percentage of families reporting good wellbeing increased from 31.8% to 43.4%, while those with poor caregiver wellbeing decreased from 38.8% to 9.6%.
Adhera Health also presented initial findings from an ongoing study sponsored by the National Institutes of Health/National Institute on Minority Health and Health Disparities (1R43MD018551-01). The presentation was titled " Adaptation of a Digital Health Intervention for Chronic Condition Related Fatigue to the Latino Population.”
This research seeks to rigorously assess the acceptability of a digital intervention within the Latinx community and devise a tailored strategy for its effective implementation in urban Latinx neighborhoods. Adhera Health conducted over 15 participatory workshops, engaging more than 150 families and stakeholders, including parent groups from diverse backgrounds such as African American and non-Hispanic white families. These sessions provided critical insights into the unique needs and preferences of the Latinx population, establishing a foundation for culturally sensitive, community-driven solutions in chronic disease management. Latinx parents of children with T1D rated the Adhera Caring Digital Program positively (77.5-88), while parents of children with obesity scored even higher (94-100). All participants would recommend the program, describing it as "easy," "understandable," and "user-friendly."
"These findings validate our mission to adopting a holistic approach that supports the entire family unit,” said Ricardo C. Berrios, Co-Founder and CEO of Adhera Health. “Our research proves that personalized digital interventions significantly enhance caregiver wellbeing, which in turn enables families to more effectively manage their child’s chronic condition, ultimately leading to improved quality of life for the child and the family as a whole.”
Adhera Health is actively scaling its family-focused model to encompass a broader range of pediatric chronic conditions and communities across the US. Its vision is to ensure that all families of children with chronic illnesses have equitable access to innovative digital health solutions that are tailored to address their specific needs and improve health outcomes.
Adhera Health at HLTH 2024
Adhera Health's team will showcase live demonstrations of the Adhera Caring Digital Program at the Scale Health booth (#3210-13) at HLTH 2024, where attendees can experience how the program empowers families managing pediatric chronic conditions through engaging and personalized support.
This blog article is a reprint of the press release - https://www.einpresswire.com/article/750051619/adhera-health-pioneers-family-focused-digital-health-solutions-for-children-with-chronic-conditions
New Study Reveals Impact of Personalized Digital Companion on Caregivers of Children with Type 1 Diabetes
Adhera Health’s AI-Precision Digital Companion Improves Wellbeing of Caregivers for Children with Type 1 Diabetes
Adhera Health’s AI-Precision Digital Companion Improves Wellbeing of Caregivers for Children with Type 1 Diabetes
Groundbreaking research on the beneficial effects of Adhera Health’s AI-Precision Digital Companion on the wellbeing of caregivers of children with type 1 diabetes and its effectiveness to support personalized pediatric care is set to be unveiled at the 46th Congress of the Spanish Society of Pediatric Endocrinology – SEEP 2024.
Led by researchers from Hospital Universitario Miguel Servet and Adhera Health and sponsored by Novo Nordisk, this study (CARING-T1D) sheds light on the transformative potential of personalized digital interventions to enhance health outcomes for families managing chronic conditions. Building upon previous findings released at SEEP and other endocrinology conferences, the study underscores the pivotal role of personalized digital companions in providing tailored support, empowering caregivers, and driving positive engagement and behavior change to better integrate care for families affected by type 1 diabetes.
Dr. Antonio de Arriba, Pediatric Endocrinologist at the Hospital Universitario Miguel Servet and the principal investigator, will present the new findings at this year’s SEEP meeting, which takes place May 8 to 10, 2024, in Las Palmas de Gran Canaria (Spain).
“The findings highlight how Adhera Health’s personalized digital companion and interventions impact the wellbeing of caregivers and children with type 1 diabetes, revolutionizing pediatric healthcare by offering targeted support, educational content, and empowering the entire family unit,” remarked Dr. de Arriba, “This study illustrates the potential of delivering precision medicine that acknowledges the uniqueness of each family, which is essential in pediatric care for all conditions.”
Results of the new study demonstrate that a digital intervention program delivered by the Adhera AI-Precision Digital Companion had a positive effect on the overall wellbeing of the parents while also supporting integrated care via advanced AI algorithms. This means that the intervention not only positively impacted the parents’ emotional and psychological state but helped clinicians better understand the needs of families.
"The wellbeing of the entire family is paramount in managing diabetes effectively," emphasized Ricardo C. Berrios, co-founder, and CEO of Adhera Health. "Our study, alongside others we've conducted, highlights the importance of involving the whole family unit in the design of interventions and treatment plans for pediatric diabetes. It's crucial for healthcare strategies to address the needs of both children and their primary caregivers to achieve better health outcomes. Our findings support that data-driven solutions can help personalize pediatric care by considering the distinct needs of each family."
The study results also show that stratifying families based on the child’s metabolic control and the wellbeing of the caregiver provides a valuable approach for assessing the overall wellbeing of the family unit. By considering these factors together, healthcare professionals can gain a more nuanced understanding of the family’s health, and subsequently tailor interventions more effectively to meet the specific needs of each family.
This blog article is a reprint of the press release - https://www.einpresswire.com/article/709927170/new-study-reveals-impact-of-personalized-digital-companion-on-caregivers-of-children-with-type-1-diabetes
National Minority Health Month - Be the Source for Better Health
Since 2002, the National Institute on Minority Health and Health Disparities (NIMHD) has annually celebrated National Minority Health Month in April. This initiative traces its roots back to 1915 with the establishment of National Negro Health Week by T. Washington, marking a longstanding commitment to addressing disparities in healthcare access and outcomes*.
The FDA defines health equity as “the attainment of the highest level of health for all people, where everyone has a fair and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography, preferred language, and other factors that affect access to care and health outcomes.” Despite strides made in awareness and policy, significant work remains to achieve this vision.
In the realm of digital health, there’s growing evidence of the positive impact digital tools can have on chronic condition self-management. However, studies indicate a concerning trend: low adoption rates among minority populations in the U.S., which often stem from poor adaptation of digital health solutions to minorities’ specific needs. This can be attributed to a systemic lack of involvement of minority groups in the design of these digital health solutions. Consequently, this is raising concerns about potential exacerbation of health inequalities.
Recognizing the potential of digital health solutions to improve health outcomes, Adhera Health prioritizes inclusiveness as a value in both our daily operations and our product development. We believe that personalized solutions tailored to the unique needs of health minorities are essential for achieving health equity. In line with this commitment, we were honored to receive a NIH/NIMHD award in September 2023 for advancing our Adhera Precision Digital Companionä platform for chronic conditions and adapting it to the U.S. Latinx population with the aim of reducing health inequalities.
Adhera Health is privileged to collaborate with Dr. Jennifer Raymond, MD, MCR, Chief of the Division of Endocrinology, Diabetes, and Metabolism at Children’s Hospital Los Angeles, on the NIH/NIMHD award. Dr. Raymond is enthusiastic about our project and is particularly eager to spearhead the clinical research on pediatric diabetes, encompassing the comprehensive care of both the physical and mental wellbeing of family caregivers of Latinx children with type 1 diabetes.
Dr. Raymond strongly advocates for leveraging digital health technologies to alleviate distress among young individuals with diabetes, particularly those residing in marginalized communities. A study published in 2023 by the Journal of Diabetes Science and Technology, led by Dr. Raymond and her colleagues, revealed that adolescents and young adults with type 1 diabetes who primarily engage in diabetes clinic visits via telehealth exhibit improved overall attendance and experience lower levels of diabetes-related distress compared to those attending in person.
To support our efforts and raise awareness, we encourage you to explore the Minority Health and Health Equity Resources Catalog, which offers a comprehensive list of resources available in multiple languages: https://www.fda.gov/consumers/minority-health-and-health-equity/minority-health-and-health-equity-resources
Together, let’s strive for a future where everyone has equal access to quality healthcare, regardless of their background or identity.
Sources:
Unlocking Insights with Kwame Ulmer: A Journey Through MedTech, FDA, and Venture Capital
Unlocking Insights with Kwame Ulmer: A Journey Through MedTech, FDA, and Venture Capital
This interview with Kwame Ulmer is part of a larger series of interviews where we discuss the intersection of healthcare and technology with subject matter experts from Adhera Health’s Advisory Board.
Kwame Ulmer leads MedTech Impact Partners (MIP), bringing over twenty years of experience evaluating medical technologies from both government and private sectors. In addition, Mr. Ulmer is a venture partner at Wavemaker Three-Sixty Health, the leading Southern-California based, early-stage venture capital firm focused on the healthcare industry. His career highlights include a 12-year tenure at the FDA in roles such as Deputy Director and Branch Chief, as well as serving as Vice President of Regulatory Affairs and Quality Assurance at Implant Direct, a subsidiary of Danaher Corporation. In addition to Adhera Health, Mr. Ulmer is a board member for Essenvia Inc. and Strados Labs Inc. He is a lecturer and researcher at the University of California, Los Angeles, and the founder of MedTech Color, a nonprofit aimed at enhancing the representation of persons of color in the medical device industry.
During your 12 years at the US Food and Drug Administration, you occupied leadership roles including deputy director and branch chief. How have the insights and challenges faced in these roles shaped your current approach at MedTech Impact Partners and Wavemaker 360 Health, specifically when advising startups on their journey through the FDA's rigorous evaluation process?
“There are a couple of ways my time at the FDA informed my decision-making afterward.” In his positions at the FDA, Mr. Ulmer was able to review a variety of products, “from medical devices to diagnostics, to digital health solutions – and a range of applications.” As Mr. Ulmer explains, the knowledge he gained at the FDA engaged him with “a sense of the core issues regarding establishing safety and effectiveness for any product and the basic processes and principles for evaluation that I could apply to a range of technologies when I left.”
Mr. Ulmer says that his time with the FDA taught him about the importance of knowing how to talk to the FDA. “What really became apparent after I left, was the impact of questions from the FDA and the activities that set off that accompany.” Companies put a lot of energy into “effectively responding to the FDA, and it really is a bit daunting.” When interacting with the FDA, “one has to be mindful, first of all, are the FDA asking the right questions, not having a whole laundry list of not critical or not germane or not necessary questions?” When you’re outside of the agency interacting with the FDA, you are working with companies “to effectively and efficiently answer their questions as quickly as possible, while using the right amount of resources in the organization and hopefully, not having the entire organization seize up.”
You have experience evaluating over 1000 medical technologies throughout your career. What patterns or trends have you observed in the evolution of medical technologies and how do these patterns inform your investment decisions at the venture capital level?
Mr. Ulmer explains how he has seen shifts in the medical technology industry over the last couple of decades.
“I’ll talk about the people and then the technology.” When Mr. Ulmer first began working in the regulatory space for medical technologies, he saw the same “teams” of individuals. “There was a leadership profile, and it was a clinician who had discovered a novel way to treat a pain point; sometimes they teamed up with a business-oriented person, and I would be sitting across the table with them talking about some safety or effectiveness issue. Now the profile is more diverse. It could be a former Google employee who found software to treat a mental health condition. It could be someone with no traditional medical device background at all who teams up with someone who came from pharma who wants to take a stab at the medical device industry.” While the type of entrepreneur has shifted somewhat, it still retains that traditional profile.
“The other thing that has changed over the years is the agency's focus and the industry's focus on all aspects of software-related issues. 10 years ago, the vice president at the time was in danger of having his defibrillator hacked. So, cybersecurity has been around for over a decade for medical devices. It is only in the last three or four years that there has been staffing for FDA experts in software, specifically in cybersecurity, so it has become one of the last elements of holistically established safety effectiveness. So, that is another megatrend: software as a medical device and software in a medical device, and the FDA having the capability to address it in terms of safety and effectiveness.”
What resources exist within the FDA, in terms of technical and technology knowledge, to address the rapid development of tech? For example, for generative AI applied to health care – how can they keep up with new advances?
“Yeah, it’s a challenge.” says Mr. Ulmer. “This is from reading the publicly available data about how the FDA is responding: number one, an unintended consequence of the pandemic is that there was a surge in staffing these for the FDA to get emergency-use authorizations through effectively.”
The staffing requirement is still there, “in the latest user fund negotiation, the FDA asked for additional staff. I think those staff are still coming online.” Specifically, explains Mr. Ulmer, “software experts are still coming online,” who can be difficult to find because those individuals have a lot of career options, especially in the private sector. “So that is a challenge. Getting that top talent who can address issues like cybersecurity.”
You have great experience overseeing various aspects of fund management, from deal sourcing to advising portfolio company management teams. What pitfalls do you observe startups taking in the MedTech space, and how do you leverage your experience to guide them?
“Over the years, I've been fortunate enough to establish relationships with the key stakeholders that are needed to build a high quality, safe, and effective medical device, med tech solution, and early on for rarely reasonable reasons, the founding team doesn’t always have access to all the experts that they need. So, a pitfall is not having, within arm's reach: the biostatistician, the clinician, the cyber expert, the biocompatibility experts – all the key team members you need to establish a proof of concept, and later safety and effectiveness. And that's obviously an issue of funding.”
Another pitfall is “companies not knowing, through really any fault of their own, not just that non-dilutive capital like NIH and NSF funding is there, but that most companies do not successfully get their grant approved on the first go around. It normally takes a couple of attempts to get that first a hundred thousand. And then for phase two, you know, for over a million dollars.”
As one of the founders of MedTech Color, you've made a significant effort to advance the representation of persons of color in the medical device industry. What challenges and gaps do you perceive that led to the establishment of this nonprofit? And how do you envision the future of diversity within the medical technology sector?
“The first gap, that was born out of my personal experience and the experience of other executives in the industry, was that it was a lonely experience – which impacted retention.” When people don’t feel a sense of community they tend to leave. “That was something I was dealing with professionally at the time. And so MedTech Ccolors originally was intended to build community, and we did that over the years. We have a network of over 43000 people from a range of ethnic groups and allies.”
In the time Mr. Ulmer spent as a consulting partner, he found that “early-stage companies with diverse teams, despite being more likely to have outsized returns, were not receiving commensurate capital – early-stage investment.” So, he helped to create a pitch competition “to serve as a magnet to attract additional capital from other sources like the NIH and the NSF, their partners for this pitch competition, but also large private companies who could buy strategically aligned companies to enter into their corporate accelerators.”
“So,” says Mr. Ulmer, “those are two examples, community and capital, that are a problem in the industry, and how MedTech Color aims to solve those problems.”
Adhera Health recently won an award specifically to address certain underserved populations in this U.S. and we understand that we still have a long road to a truly equitable future. Have you seen progress since you began MedTech Color?
“First, I think of four key stakeholders for this to be successful. One is the startups. And I'll give you a brief example. Over the last four to five months, we've seen at least five to six companies focused specifically on treating women going through menopause. That's great, the startup is trying to solve the problem there. The second element to success would be organizations in the government that could provide non-dilutive capital. Right now, there are a handful of sources of capital where you can focus on health equity. The National Institutes for Aging has a challenge where you can address health equity and receive a grand prize. And then, you have the venture capital community. If you look at just women's health investment, it's been flat, so we haven't seen a lot of activity there. And so, the fourth element is a nonprofit community and MedTech Color and MedTech Women are there to serve as a beacon for talent focused sometimes on health equity.” And while Mr. Ulmer believes these organizations are growing and thriving, “the hard truth is the venture capital dollars have not been increasing as much as they could be. And there are some early programs focused on health equity from the government side, but there's still a-ways to go for those two communities.”
Drawing from your experience as a lecturer and researcher at the UCLA Anderson School of Management, as well as your experience as a board member for the University of Virginia’s Licensing & Ventures Group, how do you see the role of academia in shaping and supporting the future of the med tech industry? And how do you ensure that the knowledge and innovations from these academic platforms transition effectively into real-world applications?
“There are two elements that I've observed for the successful translation of great ideas out of the university and into the private sector to reach patients. One is capital; larger research institutions like UCLA have wonderful innovation funds that can make significant investments to get companies to the prototype stage by de-risking regulations – sometimes even with reimbursement. Those early-stage questions that any company would need to face – they can get the capital before they even form a company through these innovation funds. So, at the University of Virginia where I went to school, there's a seed fund that plays that role. Sometimes they invest alongside angels. And then at UCLA, there's an innovation fund. So, the capital is one piece and that's the role that university should play.”
Mr. Ulmer explains further, “The other, beyond capital, is the university incentivizing professors to be able to work on these products beyond just a six-month sabbatical. This is hard,” laughs Mr. Ulmer, “but if it can be written, this should be written into the policies and procedures as to what is a factor for getting on tenure track or getting promoted; if part of the incentive for the factors that are considered to promote a faculty member could be formation of entrepreneurial projects, that would be the other major lever. And we talked about that some at the University of Virginia. How do we incentivize faculty to really be entrepreneurial? It's by saying this is a factor in your promotion.”
Why did you choose to join the Adhera Health Advisory Board and what do you see as Adhera Health’s top contributions to the current state of person-centric digital health platforms?
“Early on at the fund that I work at, and by nature of my work outside of the fund. We started thinking about megatrends, and one megatrend is personalized solutions. Some people call it personalized medicine. There are other terms, but fundamentally more and more software-enabled solutions are targeting the person, and it was really intriguing to be able to be associated with a company like Adhera Health which is focused on a personalized solution. As the company grew and established markets and patients to reach, I had a personal connection to the potential future ability to impact patients with autoimmune diseases because it has directly impacted my family. And professionally, I'm attracted to companies with diverse leadership teams, and that's immediately what I saw when I met you and got to know other members of the team,” he says, speaking to Ricardo Berrios, CEO of Adhera Health, “and I thought that that was going to be an advantage for you moving forward. So, for those reasons and many more, I'm delighted to be associated and work with you, Ricardo, and the company. There is going to be an amazing impact on patients, and I'm just delighted to be with you on this journey.”